PTEN
Phosphatase and Tensin Homolog (PTEN)
PTEN is a protein in humans that functions as a tumor suppressor and a metabolic regulator by promoting COX activity and ATP production. This intercellular protein acts as a dual-specificity protein phosphatase and a lipid phosphatase deleted on the 10th chromosome. It is expressed at a relatively high level in all adult tissues, including the heart, brain, liver, kidney, and pancreas. When PTEN is mutated at high frequency, it results in genetic aberrations consisting of mutations.
Background
PTEN mutations most commonly result in breast cancer. Breast cancer can spread when the cancer cells get into the blood or lymph system and then get carried to other parts of the body. There are many different types of breast cancer, the most common being carcinomas. This means the cancer starts in cells that make up the tissue lining organs. PTEN is one of these cells. PTEN is associated with progression of breast cancer through many ways: loss of heterozygosity, germline and somatic mutations in the PTEN gene, epigenetic silencing by methylation of the PTEN promoter, and protein interactions down-regulating PTEN transcription. People with breast cancer get treatment of many different kinds. Most women are treated with surgery to cut out cancer tissue and radiation therapy that kills cancer cells. Anastrozole is also a drug that can be taken as a hormonal therapy.
As we learned about the inflammation of carcinogenesis cells, when PTEN is mutated, the protein is unable to regulate the growth of cancer cells. When the inflammation becomes chronic, it associates with tumor development.
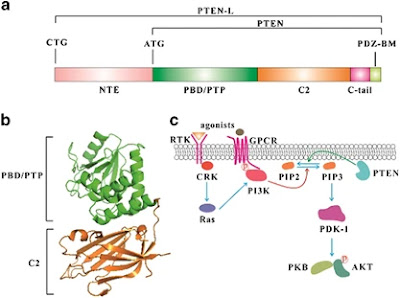
Structure: PTEN is a 403-amino acid protein that shares sequence homology with tensin and auxilin at its amino-terminal. The phosphatase domain contains the signature CX5R p-loop structure for phosphatases. The C2 domain contains the affinity for phospho-lipids. PTEN also contains two PEST domains and a PDZ domain that may regulate its stability and subcellular localization. There are several post-translational modification sites on PTEN. Two additional sumoylation sites are identified that facilitate the binding of PTEN to the membrane. When oxidation of PTEN occurs, that can lead to the formation of disulfide bonds between C71 and C124. This results in reduced PTEN activity. Clusters of phosphorylation sites are also found on PTEN.
When PTEN becomes exposed to a cancer immune microenvironment, it is no longer there to inhibit P13K. The loss of PTEN allows for an immunosuppressive microenvironment. This means an environment where there are optimal conditions for a tumor to grow. It does this by secreting immunosuppressive cytokines and MDSCs. MDSCs are proinflammatory cytokines which mean they induce angiogenesis and metastasis, thus promoting tumor growth. Fibroblasts also have a big impact on the microenvironment and the loss of PTEN from it greatly affects the environment. This causes fibroblasts to increase ECM deposition and angiogenesis.
Discussion/Future direction
PTEN plays its role in human biology. It provides instructions for making an enzyme that is found in almost all tissues in the body. This tumor suppressor helps regulate cell division and prevent inflammation. In the future, better healthcare will be able to earlier spot any mutations or growing tumors in the body. By studying these tumor suppressors and observing how they mutate, scientists gain a further better understanding of ways to fight growing tumors.


_page-0001.jpg)


